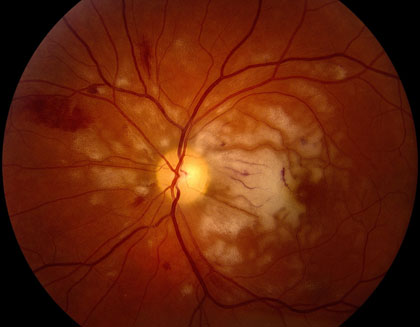
Retinal Artery Occlusion is the result of a blocked retinal artery, due to blood clotting or arterial inflammation. The condition has two types. The first is Central Retinal Artery Occlusion (CRAO), which is a blockage of the central retinal artery at the point of entry. The second is Branch Retinal Artery Occlusion (BRAO), which is usually a blockage of one branch of the central retinal artery. The latter typically causes less severe vision loss but both conditions may be a sign of a serious systemic disease.
Diagnosis
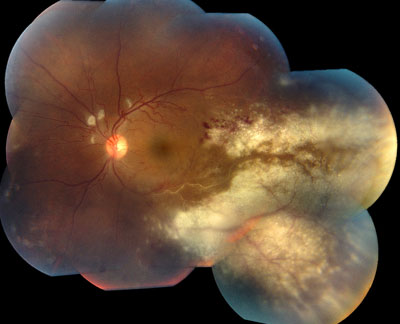
Retinal Artery Occlusions can be identified through the examination of the retina with special lenses, used to view the back of the eye. However, examinations do not always provide accurate indicators of a blockage. While disruption of blood flow, via segmentation (“box-carring”), can occur within seconds of initial vision loss, examinations may show normal results in these early stages. However, in the minutes to follow, as the retina becomes swollen from lack of oxygen, areas of retinal whitening become visible. While swelling usually resolves in weeks to months, producing clean retinal examination results, the retina might be permanently damaged.
Fluorescein angiography can reveal a disruption of blood flow and can help identify the location of the blockage. Late complications, such as abnormal blood vessel growth (retinal neovascularization) can also be identified and may be used to guide treatment.
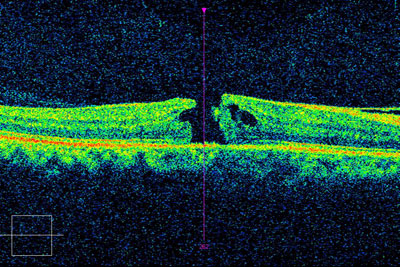
Optical coherence tomography (OCT) will show initial thickening of the retina, due to cellular edema followed by thinning of the retina, due to death of retinal tissue. Retinal thinning may be the only sign of a retinal artery occlusion, in the late stages of the disease.
Whose at risk?
Retinal Artery Occlusions are usually the result of an embolus (clot or plaque) from the carotid artery or the heart. An embolus, in either location, occludes blood flow to the retina. Because the retina is an extension of the brain, a retinal artery occlusion is considered a “stroke” of the eye. Approximately two-thirds of patients have underlying hypertension (high blood pressure) and one-fourth of patients will be found to have some preexisting disease. These diseases might include, significant carotid artery disease, characterized by the presence of plaque with a narrowing of the arterial lumen, cardiac valvular disease, or diabetes.
Some patients have one of the above diseases, combined with another underlying disorder. An extensive evaluation by an internal medicine physician may be required, using the carotid artery doppler ultrasound technology and echocardiography. Blood tests for clotting disorders (e.g., Protein S or C deficiency), elevated lipids (e.g., cholesterol and triglycerides) and inflammation (e.g., erythrocyte sedimentation rate and C-reacitve protein levels for Giant Cell Arteritis) may be required to identify risk factors in some patients.
Signs and Symptoms
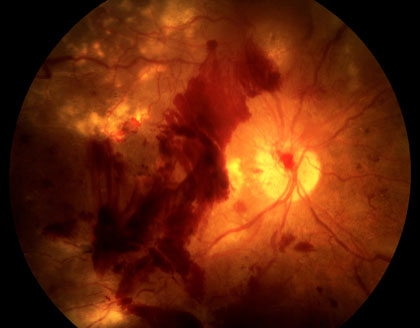
In classic cases, Central Retinal Artery occlusion presents with a “cherry red spot”, caused by diffuse retinal whitening, which surrounds and, thus highlights, a central area of redness, called the fovea. The fovea is spared from the whitening due to the lack of retinal capillaries in the center of the retina. Branch retinal artery occlusion presents with a segment of retinal whitening, which extends from a blood vessel branch point. Symptoms of CRAO usually include severe vision loss; in fact, most patients can barely count the fingers in front of their face or see light, through the affected eye. Symptoms of BRAO, on the other hand, cover a broad range. In small peripheral occlusions, there may be zero central vision loss but in more widespread cases, vision loss can be severe, located centrally and/or directly above or below the center. Both conditions may be preceded by episodes of transient visual loss, known as amaurosis fugax.
Treatment
There is a very short window of opportunity (approx 90 minutes) available for the treatment, after the onset of vision loss. However, later rescue has been reported. While no tried-and-true standard of care exists, a diversity of treatments has been utilized with some reported success. If an embolus is identified, decreasing the eye pressure or dilating the retinal vessels may disrupt the blockage and restore vision. Pressure can be decreased, by way of fluid removal, in a number of ways, including needle extraction, ocular massage or glaucoma drops. Dissolving a blood clot is another alternative. A drug called “TPA” has been administered for this purpose, via direct vessel injection or systemic injection. It should be noted, however, that there has been some reported risk of stroke with this procedure. Other affective treatment methods include, rupturing plaque with a YAG laser, breathing into a paper bag to increase CO2, vessel dilation, hyperbaric oxygen treatments and steroid use. Systematic disease treatments, such as a carotid endarterectomy, cardiac valve replacement, anti-hypertensive drug therapy, lipid lowering agents and anti-coagulants may also be suggested. If ocular complications, such as a vitreous hemorrhage or rubiosis, occur as a result of treatments, anti-VEGF drugs (eg, Avastin), laser surgery or vitrectomy surgery may be employed. New restorative treatments are now available and we specialize in those therapies here at the Retina Macula Institute.